Using the NZMT listing application form
Guide to using the application
form
1. Download the blank application form spreadsheet from the NZULM website and save it in a convenient place. You can also download it from here
2. Open the blank form and save it with a new name which reflects the application under preparation. This will help you and the NZULM editorial team to identify the file. Sponsor and contact details, notification type
3. Complete the sponsor company and contact person details by typing them into the spreadsheet cells provided:
4. Click on the notification type cell to reveal the dropdown list (1), then select the appropriate notification type from the dropdown list (2):

Selecting the correct section
of the application form to use
5. Before recording pack details select the correct section of the form to use. There are 3 sections to choose from:
● The single dose form section [(1) below]
● The multiple dose forms all of which have active ingredients section [(2) below]
● The multiple dose forms section some of which have active ingredients and others which have inactive ingredients (eg injection diluents) [(3)below]

6. Click on the appropriate link to go the section of the form.
Single dose form products
7. Use Section 1: Single dose form application of the form.
8. Begin filling in pack details by recording the trade name of the product and its active ingredient (1) or ingredients if there is more than one. Then select the appropriate dose form description from the dropdown list (2). If there is more than one active ingredient record each in the cells provided.

9. Select the appropriate pack description from the dropdown box then fill in the pack size.
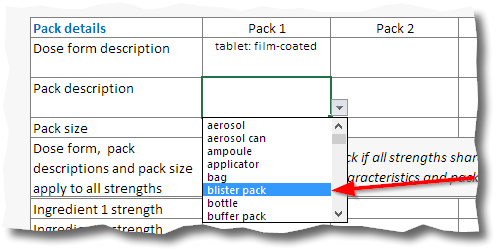
When the application covers more than one
pack
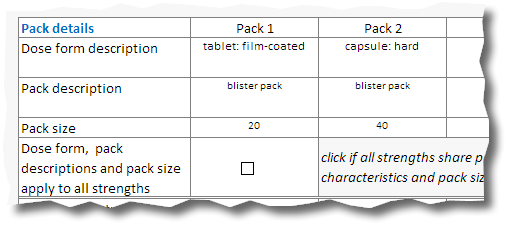
10.If the application covers more than one pack, and the dose form, pack size and pack descriptions are common to all packs tick the «Dose form, pack descriptions and pack size box apply to all strengths» box (1).If each pack is different fill in each column of pack detail cells with the appropriate information.
Common pack characteristics:

Different pack characteristics:
11. If the application covers more than one strength and multiple pack sizes for each strength it may be necessary to fill in several forms to cover all the strengths and pack sizes. To ensure listing applications are clear, if possible keep all pack sizes for a given strength on the same application form. If you are uncertain about how best to record an application using the form please e-mail the NZULM team at [email protected] or phone on 021 0232 8855.
Active ingredient details
12. Record the strength of each active ingredient for each pack size. This information is needed even if each pack shares common characteristics:
Common pack characteristics:
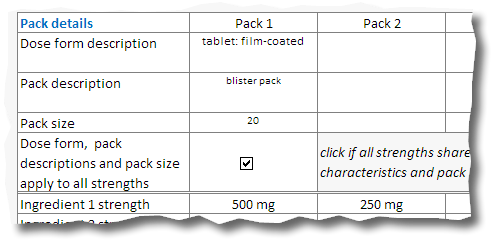
Please note: in this example listings for 250 mg x 20 tablets and 500 mg x 20 tablet blister packs are sought.
Different pack characteristics:
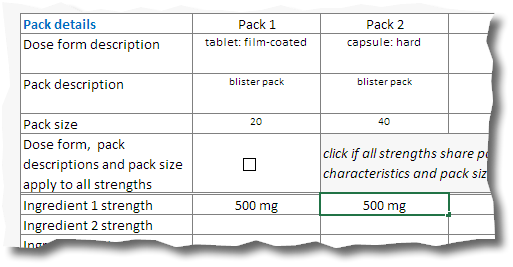
Please note: in this example listings for a 500 mg x 20 tablet pack and a 500 mg x 40 capsule pack are sought.
Multiple dosage forms with active
ingredients applications
13. Use Section 2: Multiple dose forms, all of which have active ingredients of the form.
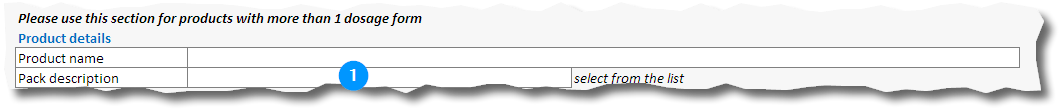
14. Fill in the product name and select the pack description (1) from the dropdown list:
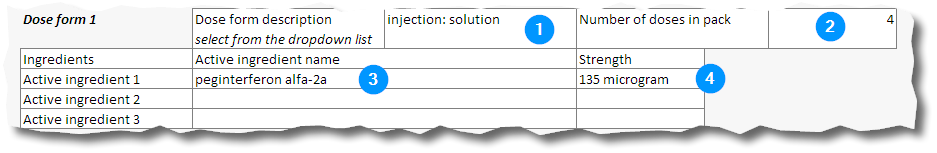
15. For each dosage form in the pack record the close form description (selected from the dropdown list) (1), the number of doses of that dosage form in the pack (2) and the active ingredient (3) and its strength (4) in the pack.
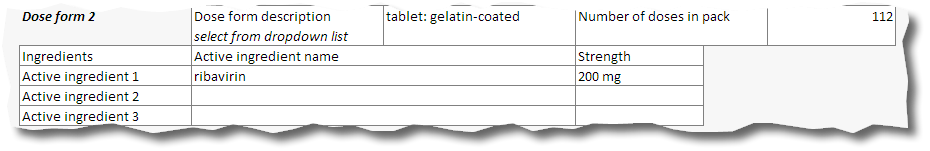
16. Repeat this for the second and any other dose forms in the pack.
Multiple dosage forms some with active
ingredients and others with inactive ingredients
17. Use Section 3. Multiple close forms, some of which have active ingredients and some have inactive ingredients of the form
18. Fill in the product name and select the pack description (1) from the dropdown list:

19. For each active ingredient dosage form in the pack record the dose form description (selected from the dropdown list) (1), the number of doses of that dosage form in the pack (2) and the active ingredient (3) and its strength (4) in the pack.

20.
Repeat this for
the second and any other active ingredients dose forms in the pack.

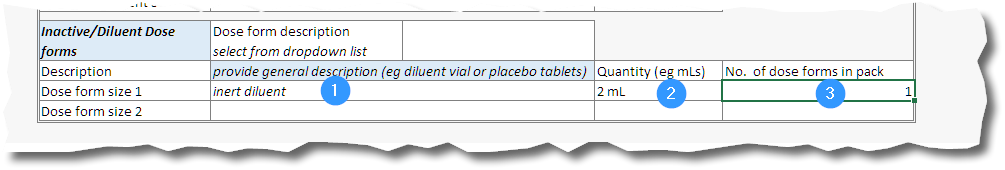
21. For the dose form with inactive ingredients, in the Inactive/Diluent Dose forms space record the dose form (selected from the dropdown list as before), provide a description of the dose form (1), the quantity in the dose form pack (2), and the number of this dose form in the pack (3). Repeat if there is a second inactive dose form in the pack.
When complete, e-mail the saved form to [email protected]
The NZULM and NZMT are services of the New Zealand Ministry of Health.
